Your cart is currently empty!
Refund
Are you looking for funding to purchase medical equipment?
We will help with all the paperwork.
Akson Hotline695 889 669.
Poniżej lista lekarzy wystawiających zlecenia NFZ:
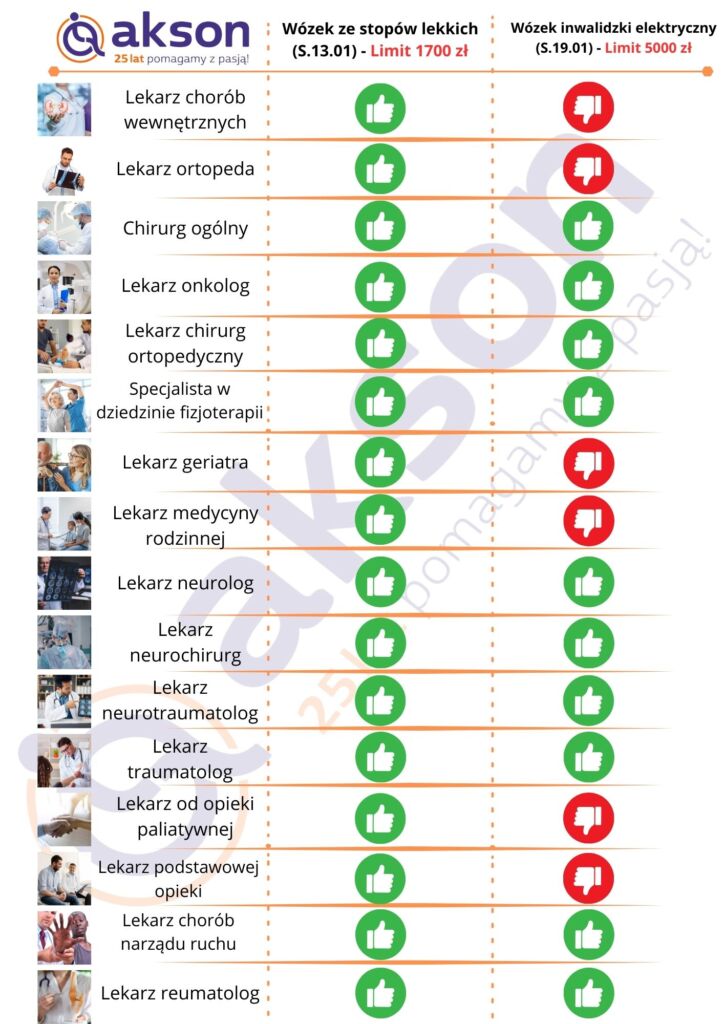
The National Health Fund (NHF) reimburses medical devices up to the limit specified in the Health Ministry’s regulation.
If the price of the medical device is lower than the limit, the patient’s deductible is calculated on this price. If the price of the device chosen by the patient is higher than the one specified by the limit, he or she will pay the difference between the gross price and the reimbursement amount.
According to the decree of the Minister of Health, the reimbursement of a medical device is granted to the patient for a specific period, and only after the expiration of this period is it possible for the authorized person to issue another order.
An exception to the above rule is the possibility of applying for a reduction in the useful life of certain medical devices, directly indicated in the Health Minister’s Decree of May 29, 2017. On the list of medical devices issued on prescription, based on the application issued by the person authorized to issue the order.
The cited regulation establishes criteria for granting medical devices whose useful life may be shortened, i.e.:
- In adults in the case of changes in the physical condition of the person, making it necessary to shorten the life of the medical device, and the possibilities of adjusting the device have been exhausted;
- In children under 18 years of age, when the possibilities of regulating the device have been exhausted, and changes in physical condition have occurred as a result:
- surgical procedures or disease entities that make it necessary to shorten the life of the medical device,
- rehabilitation,
- physical development.
A request for a reduction in the useful life of a medical device, issued by a person authorized to issue an order, must include a detailed medical justification in accordance with the basic criteria for granting the device. The issued application for shortening the life of a medical device, together with a properly issued order for a medical device, must be submitted to the Fund Branch where the Patient is registered.
Reimbursement from the National Health Fund for rehabilitation and orthopedic equipment, is available to any insured person under social and health insurance, but you can also apply for additional funds from other institutions, such as PCPR or MOPS. The process of proceeding can be found in the relevant tabs:
changes from 03/03/2018:






